In-house Sterilization of Medical Devices

With gaseous chlorine dioxide as the sterilant, it is economically beneficial to sterilize the Medical Devices in-house at either your facility or at your Contract Manufacturer. Most Medical Device manufacturers do not want to perform in-house sterilization utilizing EtO because of the liabilities of having a carcinogenic and explosive compound on site. The cost of a system, including the Damage Limiting Construction (DLC) featuring walls that are built to explode outward and Class 1 Div. 1 electrical installation, is extremely expensive. A CD system on the other hand has none of those costs or liabilities. Cycles are quick and simple being as short as a couple of hours in many cases. No special rooms for extensive aeration are required. The whole process can take place in a single sterilization chamber which can be located anywhere within your facility. Our CD gas is registered with US Environmental Protection Agency as a sterilant (Registration# 80802-1) and is able to inactivate all forms of antimicrobial life, including spores.
ClorDiSys and CD gas make it easy to bring the sterilization of medical devices in-house. ClorDiSys partners with many chamber
manufacturers and can provide custom CD Gas Sterilization Chambers based on your specific needs. Sizing can range from multiple cubic feet to
multiple pallets. ClorDiSys is easy to work with and can assist with both Cycle Development and Validation to enable the use of
CD to be as simple as possible. CD is very similar to EtO in how it is utilized, the type of devices that can be sterilized, and
the SAL sterilization levels that can be attained. An integrated UV-VIS spectrophotometer measures and controls the gas concentration
ensuring the efficacy of every cycle and allowing for parametric release. Both are gasses but CD is not explosive or carcinogenic and
leaves no harsh residues. CD cycle times are much shorter. ClorDiSys has a draft Device Master Reference file available as a guide.
Devices can be packaged in Tyvek pouches, display boxes, or final shipping containers. Final corrugated shipping containers require
a significantly more robust cycle. CD is friendly to most materials and electronics. Hand-held battery powered devices are an ideal
candidate and present a significant issue for EtO. Datwyler has also successfully performed extensive testing on various stopper components.
 An ideal application of utilizing a CD Gas Sterilization Chamber in-house is placing it at the end of a production line to sterilize your
devices in the clamshell packaging or the display boxes at the end of each shift. For higher throughput, a sterilization cycle can be
performed half way through a shift and then again at the end of a shift due to typically shorter cycles.
An ideal application of utilizing a CD Gas Sterilization Chamber in-house is placing it at the end of a production line to sterilize your
devices in the clamshell packaging or the display boxes at the end of each shift. For higher throughput, a sterilization cycle can be
performed half way through a shift and then again at the end of a shift due to typically shorter cycles.
In-house treatment costs can be as low as $5 per load.
CD Gas vs. EtO
CD gas has been providing true sterilization for medical devices for over 25 years. While EtO is a very effective sterilant, CD gas has many benefits over EtO for a variety of applications. Both are gasses which have the ability to reach all of the individual organisms to provide desired SAL levels utilizing 6-log sporicidal BI’s. The CD gas concentration can easily be measured with a built-in UV-VIS spectrophotometer ensuring the efficacy of every cycle and allowing for parametric release. Unlike vapor methods, Chlorine dioxide, being a gas, offers repeatable and consistent kill. Unlike some novel gas methods, CD has over 25 years of use since it was developed by Johnson & Johnson for sterilizing suture products, artificial joints, and implantable contact lenses.

Chlorine dioxide is the method usually recommended when devices have imbedded batteries which are not affected by CD. CD does not get absorbed into materials like EtO does, so an aeration time of under 30 minutes is typical. Chlorine dioxide is a true sterilant gas and works at true ambient temperature. The boiling point of CD is low, at -40°C. CD gas will not condense on devices. It is a surface sterilant, therefore CD gas can sterilize prefilled syringes, while maintaining drug integrity, and medical devices with complex geometries.
CD is also not listed as a carcinogen. In almost all locations, it can simply be exhausted to the environment rather than needing to be scrubbed with hazardous solutions. It is non-flammable and non-explosive at use concentrations, so it does not require expensive damage limiting construction.
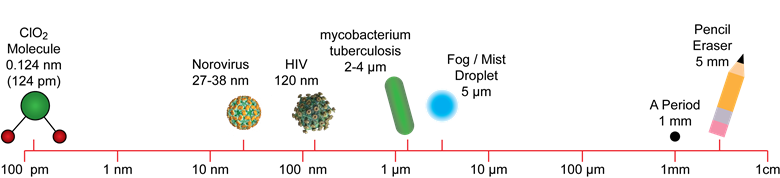
The CD Gas Advantage
Chlorine dioxide gas sterilization is ideal for sensitive biotech products and improves supply chain efficiency:
- ClorDiSys' CD Gas is an EPA registered sterilant, making it effective against all viruses, bacteria, fungi and spores
- Non-carcinogenic
- Ambient temperature sterilization (0°C - 50°C) - can be customized for unique applications
- Adjustable vacuum levels - can be customized for unique applications
- Surface sterilization process - does not get absorbed in most materials for quicker aeration
- Short cycle times - 2 to 8 hours including aeration
- Safe and easy to bring in house - reduces manufacturing time and cost
Aerates Rapidly - Minutes vs. Days
Properties as a non-vapor, surface sterilant with a low boiling point, coupled with the low CD sterilant concentrations, translates into rapid aeration of the sterilization chamber and exposed products.CD does not permeate or become absorbed in materials to the same degree as other methods, greatly reducing the overall cycle time of the sterilization process and eliminating the lengthy post-sterilization aeration.
Low Residue Levels
CD’s properties combined with the rapid aeration process results in residues below detectable levels on product and packaging. CD’s sterilant residues are non-carcinogenic, non-cytotoxic and non-teratogenic. Sterilized packages and products may be handled immediately after the cycle.
Flexible to Design Custom Cycles
| Process Parameter | Range |
| CD Gas Concentration | From 1 mg/L to 30 mg/L |
| Exposure Time | Varies with Application |
| Door-to-Door Time | 2-8 Hours |
| Relative Humidity | Typically 65-90% |
| Depth of Vacuum | 5-101 kPa |
| Chamber Temperature | Ambient |
Compatible with Medical Device Materials and Packaging
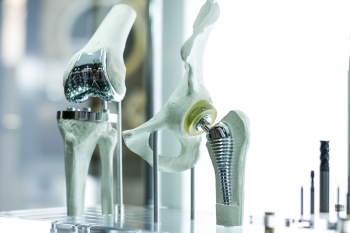
CD is less oxidative than traditional oxidizing sterilants like hydrogen peroxide, ozone, NO2 and peracetic acid. ClorDiSys has tested
many medical device materials including non-ferrous metals, polymers, batteries, tissues, bone products, and collagens. Compatible materials
showed no increase in cytotoxic response. The true room temperature performance of the CD process allows temperature sensitive materials to
be processed.
CD’s sterilization process is compatible with commonly used sterile barrier packaging. This includes: non-woven polypropylene, Tyvek® pouches,
Tyvek®-Mylar® pouches, plastic tubs with Tyvek® lids as well as cellulosic materials such as paper and cardboard.
| Compatible Materials* | |||
| Stainless Steel Aluminum Copper Glass / Ceramic Fluoropolymers Most gasket materials |
Polyethlene Polypropylene PET / PETG Polystryene Polysulfones PEEK |
Polyetherimide Polycarbonate Cycline Olefins PVC Silicone Hypalon |
Thermoplastic Elastomers Titanium Gold Platinum Cardboard Most Inks |
| *This list is not exhaustive. Specific testing with your device is always recommended. | |||
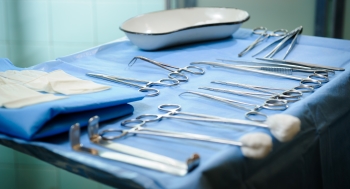
Current Applications
Below is a list of applications that CD gas has been used for. Please contact us for more information on any of these applications or to explore additional applications not listed below.
| Sample Applications |
| Implantable Contact Lenses |
| Artificial Joints |
| Suture Products |
| Bones and Bone Powders |
| Surgical Kits |
| Datwyler Stoppers |
| Endoscopes |
| Electronic Devices |
| Cold-chain Products |
| Bioabsorbable Products |
| Pre-filled Syringes |
