- Browse Methods
- Why Choose CD?
- Hydrogen Peroxide
- Steam
- Fogging
- iHP®
- Ethylene Oxide
- Formaldehyde
- Spray and Wipe
Method Comparisons
In order for any decontaminating agent to be successful, three important Principles of Decontamination must be followed. These are the underlying fundamentals which must be achieved in order to ensure a successful decontamination cycle and apply to all decontaminating agents, including hydrogen peroxide vapor. An agent's chemical and physical properties will affect the extent to which these principles are met and will influence the overall effectiveness of the decontamination cycle. Environmental factors will influence these properties and will play an important role in the effectiveness of your agent.
Principles of Effective Decontamination
Complete Distribution
In order for any sterilant to be successful, it must reach all surfaces.
Hydrogen peroxide vapor is limited by the fact that it is a liquid, and NOT a vapor at room temperature; conditions where most decontaminations take place. Hydrogen peroxide only exists in the vapor phase upon heating above its boiling point, 109°C (228°F), and is constantly condensing to its original liquid form at all conditions below this elevated temperature. Hydrogen peroxide is often referred to as a lazy gas and this "laziness" is attributed to the fact that hydrogen peroxide is not a true gaseous decontamination agent and has far less distribution capabilities than its gaseous counterparts such as formaldehyde and chlorine dioxide gas.
Total Penetration
In addition to complete distribution, the sterilant must be able to penetrate into all areas.
Similar to the points made above, any decontamination agent that is attributed to being lazy will also have reduced penetration abilities..
Concentration and Contact Time
Once your sterilizing agent has distributed to and thoroughly penetrated into all areas, it must remain there long enough to provide the desired level of kill.
Since hydrogen peroxide in the vapor phase is constantly trying to return to its original liquid form at temperatures below its boiling point, holding concentration in any area below this temperature will be extremely difficult.
Environmental Factors - Vapors are NOT Gases
Liquids and vapors, including hydrogen peroxide, are affected by many environmental factors such as temperature, room geometry and layout, and equipment loading and positioning. Injection rates and dwell times must also be calculated and validated for each unique chamber or room that you wish to decontaminate with agents of this type. Additionally, each piece of equipment added or removed or repositioned in these spaces requires new cycle development testing to be performed. On the other hand, for a true gas such as chlorine dioxide, cycle development consists of only a rough calculation of the chamber. The amount and positioning of equipment and items does not play a role in CD cycle development as gases will uniformly fill any space they are injected into regardless of environmental factors. This greatly simplifies validation efforts and easily ensures process efficacy and repeatability.
Being a true gas, chlorine dioxide observes natural gas laws meaning that by nature, it will uniformly fill any space where it is injected into. Hydrogen peroxide vapor on the other hand, is not a true gas and will start to condense back into its liquid state at temperatures below 109°C (228°F), about 20 times less than average room temperature, negatively affecting its distribution and penetration abilities. This "Vapor vs. Gas" differentiation leads to many of the differences between the two methods in terms of effectiveness and ease of use.
| Chlorine Dioxide Gas | Vapor Phase Hydrogen Peroxide | Description | |
| Distribution | Follows natural gas laws to achieve complete and uniform distribution throughout space. | Hydrogen peroxide vapor is poor at passive diffusion because of hydrogen bonding characteristics.1 | Contact is essential in decontamination, poor distribution leads to poor decontamination |
| Penetration | Able to penetrate into cracks, crevices and into some organic materials. | Unable to penetrate well due to tendency to condense on surfaces. Unable to penetrate gaps of 5mm (0.196")2 | Cracks, crevices, and gaps are commonly found in rooms and chambers. Poor penetration leads to poor decontamination. |
| Relative Humidity | Optimal range between 60-75% | Initial levels vary but final levels can reach 85%. | Increased humidity levels are essential in all spore reduction as the high Rh causes spores to swell and crack, allowing the agent to penetrate. |
| Concentration Monitoring | Integrated, validated photometric sensor which measures concentration accurately in real-time. | Chemical sensor which may be integrated at extra cost. Inaccurate concentration monitoring due to non-uniform distribution within space. | Chemical sensors can become saturated and read inaccurately. Chlorine dioxide gas is able to be photometrically measured due to its yellow-green color to provide precise measurement and control. |
| EPA Registration | Yes | Yes | Both methods are registered with the US EPA as sterilants. Product labels must be read for approved applications. |
| NSF Approval | Yes | No | Only chlorine dioxide gas and formaldehyde gas are approved by NSF International for BSC decontamination. |
- References
- 1. Orlowski, Martin. Redifining Decontamination Safety. ALN Magazine, March 2011.
- 2. Steris Case Study M1941, Industry Review: Room Decontamination with Hydrogen Peroxide Vapor. Publication ID #M1941EN.2002-09 Rev. C, Steris, 2000.
Both VPHP and CD are sterilants, which makes them dangerous by nature. Due to differences in their chemical properties and the processes used during decontamination, chlorine dioxide gas is a much safer method.
Visit our Chlorine Dioxide Gas Safety page for an in-depth look at Safety.
Depending on the size of your application, multiple generators may need to be purchased. Chlorine dioxide gas diffuses naturally, behaving according to gas laws, which allows a single CD Generators to decontaminate very large volumes. Vapor Phase Hydrogen Peroxide generators have lower volume capacities due to the vapor's poor diffusion rates which necessitates a line-of-sight injection and nearly one generator per room.
| Room Size | CD Generators Required | VPHP Generators Required | ||
| 50 ft2 | 500 ft3 | 14.16 m3 | 1 | 1 |
| 100 ft2 | 1000 ft3 | 28.32 m3 | 1 | 1 |
| 150 ft2 | 1500 ft3 | 42.48 m3 | 1 | 1 |
| 200 ft2 | 2000 ft3 | 56.63 m3 | 1 | 1-2* |
| 500 ft2 | 5000 ft3 | 141.58 m3 | 1 | 2-3* |
| 1000 ft2 | 10,000 ft3 | 283.17 m3 | 1 | 6-7* |
| 1500 ft2 | 15,000 ft3 | 424.75 m3 | 1 | 10 |
| 2000 ft2 | 20,000 ft3 | 566.34 m3 | 1 | 13-14* |
| 3000 ft2 | 30,000 ft3 | 849.51 m3 | 1 | 20 |
*Lower number of generators used if room consists of simple geometry (rectangular) and is empty of equipment.
Cycle times vary among both chlorine dioxide gas and vapor phase hydrogen peroxide generators mainly because of the difference in aeration times. Chlorine dioxide gas can aerate from a chamber in 12-15 air exchanges, normally around 30 minutes for rooms, and under 5 minutes for isolators, Biological Safety Cabinets, and HEPA housings when direct venting is possible. VPHP aeration times are lengthy because of absorption into materials and condensation on surfaces. Below are examples of published cycle times for both isolator decontamination and room decontamination.
| Isolator Decontamination | ||
| Steris VHP | ~25 ft3 | 3-6 hours3 |
| Bioquell Clarus | ~25 ft3 | 3-3.5 hours3 |
| ClorDiSys Minidox-M | 31 ft3 | 1.3 hours4 |
| Room Decontamination | ||
| Steris VHP | 300 ft3 | 7.5 hours5 |
| Steris VHP | 530 ft3 | 10+ hours9 |
| Steris VHP | 760 ft3 | 4.5+ hours and overnight aeration6 |
| Bioquell Clarus | 2500 ft3 | 10-11 hours7 |
| ClorDiSys Minidox-M | 2700 ft3 | 3.5 hours8 |
- References
- 3. Caputo, Ross A. and Fisher, Jim. Comparing and Contrasting Barrier Isolator Decontamination Systems. Pharmaceutical Technology, November 2004.
- 4. Czarneski, Mark A. and Lorcheim, Paul. Isolator Decontamination Using Chlorine Dioxide Gas. Pharmaceutical Technology, Volume 29, No 4, April, 2005.
- 5. Steris Case Study M1456, VHP Case Study #1 Hydrogen Peroxide Gas Decontamination of A Material Pass-Through (MPT) Room, Publication ID #M1456(8/99), Steris, August, 1999.
- 6. Steris Case Study M1455, Case Study #3 - VHP 1000 Decontamination of a 760 ft3 room Containing Blood and Urine Analyzers, Publicaiton ID#M1455/990810 (8/99), Steris August 1999.
- 7. Room Decontamination Presentation to Council on Private Sector Initiatives, Washington, DC, by Henry Vance PE of Alpha Engineering, February 11, 2002.
- 8. Lorcheim, Paul. Decontamination using Gaseous Chlorine Dioxide, A case study of automatic decontamination of an animal room explores the effectiveness of this sterilization system. Animal Lab News, Vol. 3, No. 4 , p25-28, July/August 2004.
- 9. Rogers James., Choi Young W., and Richter, William R., "Effects of Drying and Exposure to Vaporous Hydrogen Peroxide on the Inactivation of Highly Pathogenic Avian Influenza (H%N!) on non-porous Surfaces", Applied Biosafety Vol. 16 No. 1, pp4-8, 2011.
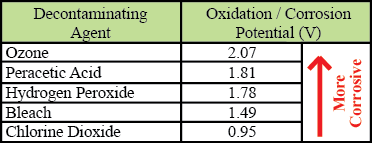
Both chlorine dioxide and hydrogen peroxide are oxidizers. Corrosiveness can be quantitatively measured by examining the chemical's oxidation / reduction potential. The higher a chemical's oxidation / reduction potential is, the more corrosive it is. As can be seen in the table below, chlorine dioxide is less corrosive than both bleach and hydrogen peroxide.
Please visit our Chlorine Dioxide Material Compatibility page for complete information.
Download our Chlorine Dioxide Material Compatibility Brochure for more information.
